|
Acid rain is, like it sounds, rain containing acidic
materials. It is created by acid being burned or evaporated into
the sky, and then contaminating the clouds. Then, when the clouds
rain, the acid rains on to the earth, causing damage to soil, lakes,
and even materials on books and century old paintings. This acid
cause cause great damage when it goes into soil or lakes, because
it kills many organisms that try to live there. Here is a diagram
of how acid rain is created:
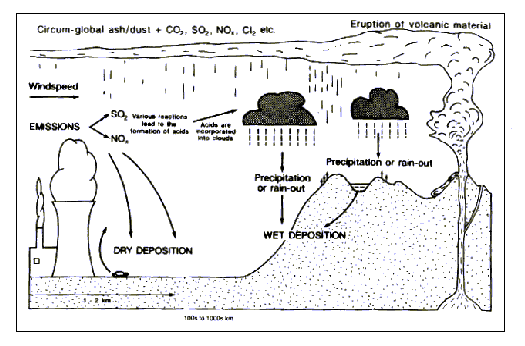
Acidity is measured using a pH scale, with the number
7 being neutral. Consequently, a substance with a pH value of less
than 7 is known as acidic, while one of a value greater than 7 is
basic. It is also important to note that the pH scale is logarithmic,
that is, a substance of pH of 6 is 10 times more acidic than another
with a pH of 7. Generally, the pH of 5.6 has been used as the baseline
in identifying acid rain, although there has been much debate over
the acceptance of this value.
The following is a visual illustration of the pH scale:

Interestingly enough, a pH of 5.6 is the pH value
of carbon dioxide in equilibrium with distilled water. Therefore,
acid ran is defined as any rainfall that has an acidity level beyond
what is expected in non-polluted rainfall. In essence, any precipitation
that has a pH value of less than 5.6 is considered to be acid precipitation.
|

